A single crystal of aluminum oxide, α-Al2O3, which has a stable corundum structure at high temperatures, is called sapphire. There is also a spinel-type γ-Al2O3, which is commercially available as a raw material or reagent for sapphire crystals.
Sapphire has a trigonal (rhombohedral) crystal structure, but is generally treated as a hexagonal crystal, as shown in the figure below.
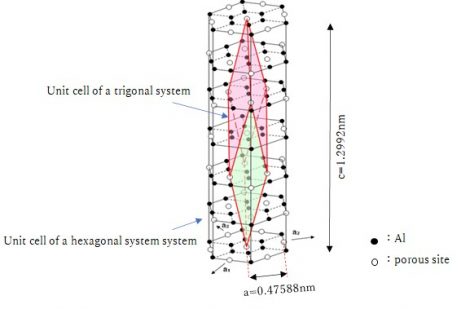
In epitaxial thin film growth, the lattice mismatch is used to quantify the matching between a substrate and a film. The lattice mismatch is defined as (|af-as|)/[(af+as)/2] (af, as : the lattice constants of the film and the substrate), and the smaller the value, the better the lattice matching and the less likely it is that lattice distortion will occur between the substrate and the film, resulting in a high-quality film. In the case of sapphire crystals, the lattice constant “a” corresponds to the distance between sites along the a-axis, as shown in the figure above, because it is considered as a hexagonal unit cell. However, in reality, epitaxial growth does not necessarily occur along this direction, so it is necessary to consider this in accordance with the structure of the film to be deposited.
For example, when a GaN film is deposited on c-plane sapphire, it grows along the c-axis, but it is known that the a-axis of sapphire and the a-axis of GaN have an angle of 30°. Therefore, it is recommended to consider the lattice matching in the direction of Al-Al in the above figure when depositing GaN films. In other words, if we assume that the lattice constant of sapphire is 0.47588/√3 = 0.2747nm, the lattice mismatch with GaN (a = 0.319nm) is 14.9%.
Generally, there are many cases where the film is deposited at high temperatures and the operating environment is room temperature. In such a case, it is desirable to consider and discuss the thermal expansion coefficient as well as the lattice mismatch, which is also important.
Sapphire has been loved as a gemstone since ancient times. When sapphire crystallizes in the natural world, the speed of crystallization varies between fast and slow depending on the environment and impurities. As a result, it is surrounded by crystal habits that reflect its crystal structure. However, before the widespread use of X-rays, it is said that crystal structures and growth mechanisms were understood based on crystal habits.
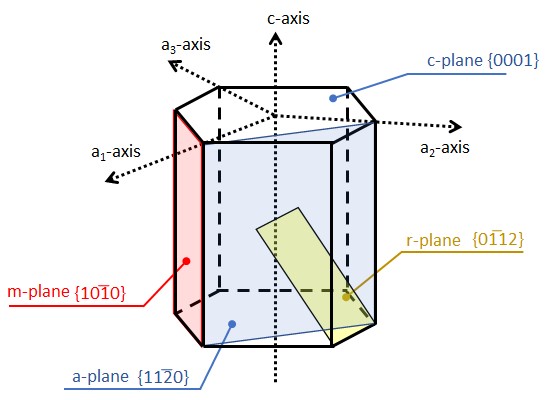
With the development of X-ray diffraction technology and crystal processing technology, the Miller index became widely used, and nowadays, the expression of plane orientation such as {11-20}, {0001}, {10-10}, and {01-12} is commonly used, but there are still terms such as a-plane, c-plane, m-plane, and r-plane derived from crystal habits.
Since there are some cases in which different names for planes derived from crystal habits are used in upper and lower cases, such as r-plane {01-12} and R-plane {10-14}, it is desirable to use them together with the Miller index notation to avoid misunderstanding.